by Frederick B. Exner, M.D.
1961
reprinted from Aqua Pura, January, 1966
In 1955, when I wrote the article which is now Chapter 4 of “The American Fluoridation Experiment,”
(1) I knew in a general way that industrial pollution of air and water with fluoridation provided a strong motive for promoting fluoridation of water supplies. But I knew few of the details, and had no idea how strong the motive was. I knew far more when I testified to the Councils of the American Medical Association, in August 1957;
(2) but the picture was far from complete. It is now clear that the one utterly relentless force behind fluoridation is American “big industry,” and that the motive is not profit, as such, but fear. Around 1900, the very existence of the smelter industry, both in Germany and Great Britain, was threatened by successful suits for fluorine damage and by burdensome laws and regulations.
(3) Today that same threat hangs over the the bulk of American big industry; and fluoridation offers both camouflage and scapegoat. Hence the relentless and uncompromising drive for universal fluoridation. The Opportunists Other promoters are more prominently involved, while Industry stays discreetly in the background. But the others are opportunists who have climbed on the band-wagon. It isn”t their band-wagon, and it never was.
Self-interest makes strange bed-fellows. Even the communists climbed aboard; but it never was their wagon.
An address by F.B. Exner, M.D. to the Western Conference of Natural Food Associates Salt Lake City, Utah, October 27, 1961
The motives of the opportunists are not always clear; but we know that basic motives usually hinge on money, power, fear or sex. Here we can rule our sex; but we can”t rule out bribery, blackmail, intimidation, greed, or lust for power. With literally billions of dollars at stake, we can know that all these play a part; but we can”t know when or where. We can rarely be sure of our own motives, much less those of others.
P.H.S. and A.D.A. A good cross section of the forces behind fluoridation can be found in a booklet called “Our Children”s Teeth,” (4) put out by a group calling itself the Committee to Protect our Children”s Teeth. In it we find promotional statements by seven officers of the U.S. Public Health Service; and three by men from the N.Y. State Health Department, which is, of course, heavily subsidized by PHS. For these people, fluoridation means money, jobs and power — such power as they had only dreamed. When you can drag people against their wills, and when public safety is not involved, there is no limit to what you can do, or to whom.
Other statements are by men from the National Academy of Sciences (also heavily subsidized by PHS), Harvard (which, in 1960, received over $7 million in research grants from PHS), and the American Dental Association.
It is well known that the ADA is one of the most active promoters of fluoridation. It is not so well known that the promotion comes from a small clique which has engineered the “endorsements” and pretends to speak for all dentists. Neither is it known that ADA received $78,000 from PHS in 1958, and $109,000 in 1960. (5)
The Kettering Laboratory The other statement is by a man who needs special mention. He is Dr. Robert Kehoe, Director of the Kettering Laboratory, of the University of Cincinnati Department of Preventive Medicine and Industrial Health. He is also Medical Director of the Ethyl Corporation; and a consultant to the Tennessee Valley Authority, the Atomic Energy Commission, the U.S. Air Force, and the Division of Occupational Medicine of PHS. (6)
Economic Motives The Kettering Laboratory originated in 1925, from gifts by three industrial concerns. Its present organization dates from 1930; and, as of 1955, was the largest organization of its kind outside government circles in the world. Its specdific purpose is to investigate chemical hazards that develop in American Industrial operations. (7)
In 1955, its staff numbered about 120. Its budget was $643,000, about 90 per cent of which was from private industries and most of the rest from government agencies. (8)
Its contracts with employing firms stipulated that no “confidential” information obtained could be released without the consent of the firm that sponsored the particular project. (9) Also, policy has been to avoid situations wherein the Laboratory might find itself “on both sides of a controversy in a court of law.” (10)
Kettering Lab and Fluorine The Kettering interest in fluorine began in 1931, in connection with the refrigerant gas, Freon 12. It has expanded to cover fluorine hazards in the manufacture of aluminium and steel, the petroleum industry, and casting of magnesium. (11).
The character of its approach to such problems is exemplified by statements of Dr. Kehoe and of Dr. Francis Heyroth who, until he died, was the Laboratory”s toxicologist and its Assistant Director. He was also on the Ad Hoc Committee of the National Research Council that endorsed fluoridation.
In 1955, Dr. Heyroth testified under oath that: (a) How soluble is sodium fluoride is not a question of how much will dissolve, but of how much will dissolve and go into the urine; (b) That the fluorine concentration in the water is always equal to the concentration in the urine; (c) That we (at the Laboratory) know exactly by our experience what the usual consumption of water is; and that it is about a quart a day; (d) That any man who drank a gallon of water a day would not live long because he would soon die of water intoxication; and (e) That six parts per million would be a safe level of fluorine in a water supply. (The maximum tolerance set by PHS at the time was 1.5 ppm). (12).
And in the aforementioned booklet, “Our Children”s Teeth,” Dr. Kehoe says: “The question of the public safety of fluoridation is non-existent from the viewpoint of medical science. There is a wide margin of safety in connection with the use of water . . . which contains fluoride in concentrations of the order of 1 part per million. The concept of toxicity as a function of concentration . . . is a fairly recent one. Because interest in physiology is highly specialized, rather than general, we find this scientific truth only partially understood.” (4) (Emphasis in the original).
This, of course, is nonsense, but nonsense with a purpose.
The Lists of Names The same publication contains three interesting lists of names. One names some 300 members of the committee. Here we find the dupes, the pretenders, the do-gooders and the me-tooers, plus a scattering of those with axes to grind.
The other two lists are more interesting. They list, respectively, 229 people described as “leading American Authorities on Nutrition;” and 131 described as “The Nation”s Foremost Chemists.”
How some rated such listing, and why others didn”t, is a good question; but that is not the point. The real question is why anyone with any self-respect would permit his name on either list.
The names are appended to two statements neither of which could be honestly signed by any intelligent layman, much less by any scientist who valued his scientific reputation. I have told why elsewhere, and won”t repeat. (13)
Of course we don”t know how many actually signed. In the case of the chemists, I wrote each one and asked whether he had signed and whether he believed the statement true. Some denied signing. Some had signed without reading. Some had signed knowing the statement to be false but because they thought fluoridation so desirable that any means were justified.
In any case, not one of those on either list has, to my knowledge, repudiated the statement or demanded that his name be withdrawn.
The Subsidies In 1958, the Director of the National Institutes of Health, a part of PHS, was quoted as telling a news conference: “Inevitably, the influence NIH and the Public Health Service exert on the nation”s health problems has grown tremendously. Thousands of doctors now depend on NIH grants for most of their support. The training of many researchers is financed by the government. A majority of medical schools admit that they would be in difficult straits without government grants.”
This simple statement of fact can also be read as a thinly veiled threat or proffer of bribe. In any case, it is interesting that 201 of the 360 “chemists” and “authorities on nutrition” worked for 87 institutions, mostly colleges and universities, that received a total of more than $151 million as “research grants” in 1960. What is more, 61 on the lists received personal grants totaling almost $2 million. (5)
Names from Industry The connections for many on the lists are not self-evident and I haven”t traced them. However, 22 signers are from leading drug manufacturers. This is not too surprising since PHS has complete and arbitrary power of life or death over every drug manufacturer. They would not dare oppose fluoridation; but I wonder if they are forced to promote it. In any case, some didn”t.
Next we have 34 names from leading chemical companies, including 8 from Dupont, 7 from Merck, 5 from American Cyanimid, 2 each from Armour and Carbide and Carbon Chemicals, with one each from Allied, Harshaw Chemical, International Minerals and Metals, Minnesota Mining and Manufacturing, and others. Each I have named is deeply involved in the manufacture or use of fluorine chemicals. To these, for reasons that will appear later, we must add three names from the atomic energy installation at Oak Ridge.
Next we have three names from the Petroleum industry; and one of the men from Dupont was from its petroleum lab.
And finally we have 11 names from the food industry.
Beyond any doubt, many signed in all innocence. People have signed petitions asking that every signer be hanged. Moreover, it is easy to believe what you want to believe, however fantastic, and in each case we have mentioned either the man or his employer had compelling reasons for wanting people to believe:
(a) That water is the chief source of fluorine;
(b) That fluorine from other sources is unimportant;
(c) That the body needs more fluorine than it can get without fluoridation, and
To understand that motive, we must review some history.
Historical Background There has been fluorine poisoning as long as there have been plants, animals and people — unrecognized as such, of course, and mostly associated with volcanic phenomena or fluorine-bearing waters. Came the industrial revolution, and things were different. There came a wholesale pollution of air and country-side with fluorine fumes and fallout; and fluorine poisoning became an important industrial hazard.
There were many sources, including glass, brick, enamel and ceramic tile; but the worst offenders were the iron and copper smelters. The first recognized effects were on vegetation.
According to Ost (3), the Freiburg smelters, in Germany, first paid damages to injured neighbours in 1855; and in 1893 had paid out 880,000 marks for current injuries and 644,000 for permanent relief. Around the turn of the century, as I said before, the situation became acute, and the very existence of the smelter industry, both in Germany and Great Britain, was threatened.
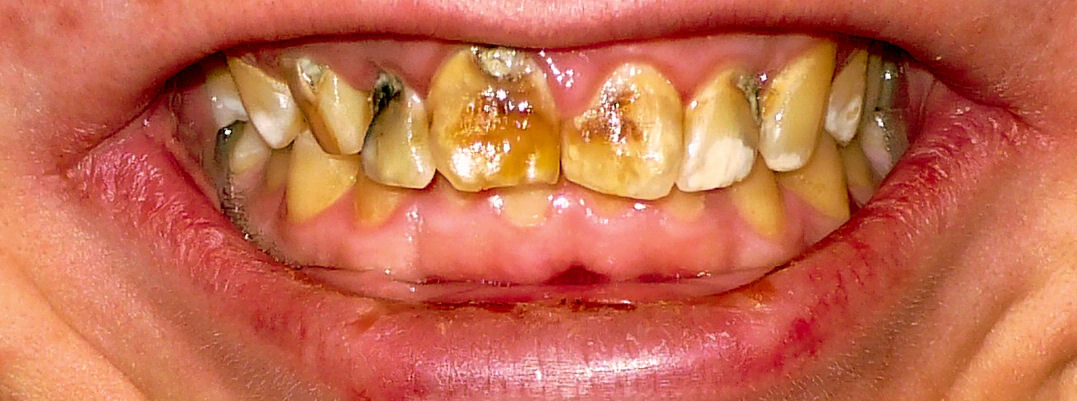
Meanwhile the cattle around Anaconda, Montana, developed what were known as “copper teeth,” remarkably similar to the human disease which became famous as “Texas teeth,” “Colorado brown-stain,” or “mottled enamel” and was later identified as fluorine poisoning. (14) I find no description of the other effects on the cattle; but we know that a cow with mottled teeth is a poisoned cow, just as a child with mottled teeth is a poisoned child. Neither will ever be as well as if it hadn”t happened.
In any case, it wasn”t to preserve the beauty of the teeth of cows that the enormous stacks were built at Anaconda, Great Falls, and Tacoma. It was to carry fluorine and arsenic high into the upper air.
Then in 1907, a disease of cattle that had been endemic around Freiburg for some 20 years was identified as fluorine poisoning from the smelters. (3)
Superphosphate and Aluminium Then came two new industries which were in immediate trouble. During the 90”s there had been numerous complaints of damage to vegetation around superphosphate fertilizer plants. In 1912, Bartolucci reported fluorine poisoning of cattle near a superphosphate plant in Italy. (15)
Between 1911 and 1918, the cattle around a Swiss aluminium plant became poisoned. The disease was identified as fluorine poisoning by Christiani and Gautier; but the Court was not convinced and damages were awarded for injury to the land but not to the livestock. (16)
During the 20”s, there was growing concern abroad, and in our own department of Agriculture and Bureau of Mines over fluorine as a public hazard — but not in the Public Health Service. PHS was under the Treasury Department; and Andrew Mellon was Secretary of the Treasury. During all those years I can find no mention of fluorine in Public Health Reports, the official publication of PHS.
Water-borne Fluoride Meanwhile there had been a parallel development with respect to teeth. There had been fluorine-mottled teeth as long as there have been people but, again, the cases were scattered and rare except in volcanic regions. With our westward migration, however, arid and volcanic regions were settled. Deep wells in the former, and even surface sources in the latter, often contained fluorine; and mottled enamel in such places was endemic.
Then Eastern communities, seeking sources of pure water, turned to deep wells, many of which contained fluorine. As a result mottled enamel which had been endemic chiefly in Colorado, Texas, and Arizona, became a problem in the East as well.
When it was discovered, in 1911, that mottled enamel was due to fluorine poisoning of the tooth-buds while the teeth were forming, PHS had to recognise that fluorine had public health importance. Dr. H. Trendley Dean was delegated to determine how much fluorine might safely be permitted in water supplies. But PHS showed no slightest interest in fluorine except in water.
Agriculture, 1933 In 1933, Dr. Lloyd DeEds, Senior Toxicologist with the Department of Agriculture and Lecturer on Pharmacology at Stanford, published a 60 page review on chronic fluorine poisoning. He wrote: (17)
“Only recently, that is within the last ten years, has the serious nature of fluorine toxicity been realised, particularly with regard to chronic intoxication. It is from the viewpoint of chronic intoxication that fluorine is of importance to the public health. A review of the literature shows that the public health aspect of fluorine is manifested in industrial hygiene, in agriculture, and in foods. The latter aspect of the problem is particularly important because of the recommendation and increasing utilization of fluorine compounds in agriculture.”
In this connection, we should note that, as of 1957, the FDA tolerance for insect-spray residues allowed 7 parts per million of fluorine in some 60 fruits and vegetables. (18) De Eds also wrote:
It is a well established fact that chronic intoxication may manifest itself in man as recognised abnormalities only after constant, or at least frequent, exposure over many years.”
And he quoted a paper by Sollman, Schetter, and Wotzel (19), saying:
According to information obtained from the United States Bureau of Chemistry, “Phosphate rock” is used to some extent in the production of phosphates used in the manufacture of baking powders. Ordinarily, such rock contains from 0.5 to 15 per cent of fluorine. The finished baking powders are made from acid calcium phosphate containing in the neighbourhood of 0.04 per cent of fluorine; but if carelessly manufactured, i.e., if calcium acid phosphate is used they may contain as much as 0.5 per cent.”
Dr. E. W. Schwartz calculates . . . that the daily intake of fluorine through the use of baking powders would approximate 0.35 to 2.84 mg. if the powders contain 0.04 per cent of fluorine; or 4.45 to 35.55 mg. if the powders contain 0.5 per cent of fluorine.
These amounts, 0.35 to 35 mg. per day, should be compared with the figure of 0.3 mg. per day that the fluoridators tell us in the average total diet, and with the 20 mg. per day that they tell us is needed to produce systemic poisoning.
Rock-phosphate is also the source of calcium or phosphorous used in many drugs and mineral supplements. In 1957, Feltman and Kosel (20) found from 1 to 286 micrograms of fluorine per tablet or capsule in 34 of 38 leading vitamin and mineral supplements. This accounted for the surprisingly high levels of blood-fluorine among patients on fluorine-free water. DeEds also wrote:
“The possibility of fluorine hazard syhould . . . be recognised in industry where this element is dealt with or where it is discharged into the air as an apparently worthless by-product.”
He discussed poisoning of vegetation and livestock near aluminium plants; and pointed out that superphosphate plants were pouring 25,000 tons of fluorine into the air and adding 90,000 tons to the top soil each year. He added:
“This sizeable quantity gives pause for thought of the potential toxicities connected herewith.”
Systemic Poisoning in Man Such was concern over fluorine in 1933; and DeEds did not yet know that Moller and Gudjonsson had already found, and described, chronic fluorine poisoning among Danish cryolite workers. In 1937, Kaj Roholm published his monumental study of chronic fluorine poisoning, which is still regarded as a classic. (21, 22)
Also in 1937, Shortt and co-workers, in India, reported poisoning like that described by Roholm and by Moller and Gudjonsson but caused by water-borne fluorine. (23)
Cox to the Rescue Concern over fluorine as a public health hazard was definitely getting out of hand. For one thing, industry was dumping its fluorine wastes in rivers — rivers that were used downstream for water supply.
This was the situation when Dr. Gerald Cox, from Mellon Institute, suggested (24), in 1939, that (and I quote):
“. . . the present trend toward complete removal of fluoride from water and food may need some reversal . . . ”
And suggested that fluoride be added to water supplies as a means of reducing tooth decay.
The PHS “Tolerance” The result was that, in 1942, instead of forbidding the dumping of fluoride in water supplies, PHS set 1.0 ppm of fluorine as the maximum tolerance in a public watger supply. Then, in 1946, and with no new “evidence of safety” it was raised to 1.5 ppm.
Again, in 1961, it had been raised to 2.4 ppm, in spite of the fact one PHS investigator (25) had said that, at 1.5 ppm, the factor of safety was zero; and another (26) had said that above 2.0 ppm the permanent disfigurement of many of the users far outweighs any hypothetical benefit.
However, the situation was desperate, because far higher levels of contamination were taking place. For example, the Peace River, from which Arcadia, Florida, takes its water, often has up to 17 ppm of fluorine caused by the triple-superphosphate plants in the river basin. (27).
A lawyer for a leading copper company told a friend of mine that Salt Lake City would be fluoridated whether the people like it or not. “How else,” he said, “can we get rid of our fluorides?”
The Blackout Since 1937, the foreign medical literature has contained hundreds of articles on the wide variety of troubles that can be caused by fluorine. The same is true of the veterinary literature in this country. But none of this appears in our medical literature.
The things that fluorine can do to people are seen every day everwhere. The trouble is to know which cases are actually caused by fluorine.
There has been constant danger that someone would analyse tissues in both high and low fluoride areas and find that fluorine poisoning is common. But if every community can be fluoridated there will be no fluorine free areas for comparison.
Meanwhile, such information (or rather misinformation) as has been disseminated in this country has come from the Kettering Laboratory, the Public Health Service, and sources they control.
All reports based on PHS research grants are subject to censorship before publication. (28) Whatever is found is so reported as to conceal any possible hazards from fluorine. A good example is the report of the PHS sponsored study at Provo, Utah, where valuable data and findings were grossly misrepresented in reporting. (29)
The result is that the physicians and dentists of America know almost nothing about fluorosis (chronic fluorine poisoning); and most of what they think they know isn”t true. Most, in fact, don”t even know there is such a thing; and because they don”t, it never occurs to them to look for it, or even consider it.
World War II In 1942, with the Second World War, there was an enormous increase in fluorine pollution. Steel production expanded; and aluminium which had been used for pots, pans and a few appliances, was needed for an air fleet. Moreover, these industries invaded parts of the country that were not used to fluorine polluted air — for example the steel plants in California and Utah, and aluminium factories in Washington and Oregon. Crops and live stock suffered, and people didn”t like it. Even the deer in the hills around Provo, Utah, had mottled teeth.
At Provo, after the war, some $30 million in damage suits were filed (30); and some $4.5 million were awarded in settlements out of court. (31) Then, about 1950, the company spent $9 million on air cleaning equipment which requires, among other things, the use of 40 tons of lime dust a day. They say this removes 90 per cent of the pollution (31); and a lawyer for U.S. Steel bragged to me that the Geneva plant is now the cleanest steel mill plant in the world. (In Pittsburgh they don”t have cattle. They only have people).
The situation regarding aluminium was much worse. Aluminium is made by electrolysis of bauxite (aluminium oxide) in a bath of molten cryolite (sodium aluminium fluoride), either artificial or the natural mineral.
In a typical plant, with four “pot lines” of 128 “pots” each, five tons of fluorine (as cryolite, aluminium fluoride, and calcium fluoride) were added each day to replenish losses. Of this, the company estimated that 7,000 pounds a day escaped into the atmosphere. (32)
This plant, at Troutdale, Oregon, was built and operated for the Government by Alcoa during the war. In 1946, it was rented from the Government by Reynolds Metals who demanded that air-cleaning equipment first be installed. This was done at a cost of more than $270,000. This cut the emission to less than 4,000 pounds per day.
Additional controls were installed in 1950, at a cost of more than $2 million, and cut emission to less than half a ton per day.
Meanwhile, some millions in damage suits were filed; and many hundreds of thousands paid in settlements or judgements. One suit, for damage to the members of the Paul Martin family, is the only successful suit for damage to humans by fluorine pollution in the United States to date. Alcoa, Kaiser, Harvey Aluminium, Olin-Mathieson, Victor Chemical, and Food Machinery and Chemical, all joined in the suit as “friends of the Court.” (32, 33)
Practically all the medical testimony for the Company came from four men from Kettering and one formerly from Kettering.
The story elsewhere is similar — at Sauvie Island, Longview, Tacoma, Spokane — with extensive damage to crops, land, and live stock.
At the Dalles, Oregon, vegetation was analysed both before and after the plant was built. The plant was opened July 26, 1958. On June 30, the average fluorine content of seven crops grown within a mile of the factory was 3 ppm. After 73 days of factory operation it had jumped to 140 ppm. The following year, peaches contained up to 22 ppm of fluorine; and many suffered from the condition called “soft suture.” (34)
At Longview, the people voted down fluoridation in 1952. A few years later, children started to show mottled teeth (35); whereupon the Council put in fluoridation without a vote. Now the mottling can be blamed on the water rather than the aluminium plant.
Petroleum Industry World War II also brought new sources of fluorine pollution. The Kettering Laboratory has compiled and published abstracts of some 8,600 articles on inorganic fluorides up to 1958. It contains 639 articles on uses of fluorine compounds in industry. Of these, 76 were published before 1942, and 563 since. Most of the latter deal with new uses of fluorine compounds, and new sources of pollution.
One major change was the substitution of hydrogen fluoride for sulphuric acid as catalyst in the production of high test gasoline. According to Callaham (36), one such plant required 500 to 750 tons of hydrogen fluoride yearly. How much of this goes directly into the atmosphere, and how much remains in the gasoline to appear in car exhausts, has never been told.
In any case, the first such plant was put in operation in Los Angeles in 1942; and by a strange coincidence, that was the year of the first complaints of eye irritating smog. Eye iritation is also the first noticeable effect of hydrogen fluoride for most people.
For several years the Los Angeles papers told about the hydrogen fluoride in the smog; but by the time the reports reached Seattle, fluorine wasn”t mentioned. Now it isn”t mentioned even in L.A. and we are told there is no fluoride in the L.A. smog. This is strange since there is fluoride in the air of every major city, with or without smog.
Now we are told that the eye-irritation is caused by hydrocarbons, ozone, or oxides of sulphur or nitrogen, all of which could be smelled strongly if in eye-irritating concentration.
Since 1942, numerous other uses of fluorides in petroleum refining have been added; and both hydrogen fluoride and the highly toxic boron trifluoride are used.
Elemental Fluorine Fluorine itself, the chemical element, is the most reactive of substances; and does not occur in nature. It combines rapidly and violently with whatever it touches except its own compounds.
Prior to 1942, it was made with great difficulty, in gram quantities; and could not be bought at any price. The problem of containing it was solved by treating materials to form a tight surface coating of fluoride. This protects the underlying material from further attack. (37)
It is now shipped in tank trucks of 5,000 lb. capacity. The consequences of a wreck are not pleasant to contemplate. There would be no explosion, but it would consume everything it touched, including water, steel, concrete, and people. The heat would be terrific. The products of combustion would all be poisonous, and most of them corrosive. Enough poison to kill a million people would result; and decontamination would be a major undertaking.
Fluorine is also being tried as a rocket propellant. With hydrogen or hydrazine as fuel, it makes the most effective chemical propellant that is possible. We are told (37) that it creates no toxic hazard, but this is hard to believe.
Atomic Energy Enormous quantities of fluorides are also emitted in the refining of uranium. Uranium 238 is separated from its lighter isotopes as Uranium hexafluoride. To make this, pure uranium oxide is treated first with hydrogen, then dry hydrogen fluoride and finally with elemental fluorine.
Uranium hexafluoride is an extremely poisonous and corrosive gas and very hard to handle. On contact with moist air, it forms hydrogen fluoride and a dense cloud of uranium oxyfluoride, both poisonous.
“UF6 was almost constantly evolved, forming clouds of smoke which frequently were so severe as to obscure vision in the plant. The only fatalities occured in the early days of production. The persons concerned exhibited symptoms of HF poisoning.” (38)
Coal Finally we must not forget coal as a source of atmospheric fluorine. According to Churchill (39), coal from Western Pennsylvania contained 85 ppm of fluorine, from Illinois 167 ppm, and from Utah up to 293 ppm.
The amounts may seem small as compared with rock phosphate; but ifwe assumea conservative average of 120 ppm, the coal mined in 1959 (40) contained over 50,000 tons of fluorine. Nearly all of this was given off in burning. (41)
The Death-fogs; The Meuse Valley, 1930 During the first week of December, 1930, all of Belgium was blanketed by dense fog. In addition, there was a temperature inversion in the Meuse Valley, which served as a lid to prevent the upward escape of gases.
In a 15 mile stretch of the Valley, with hills of 250 to 350 feet on each side, some 6,000 people became violently ill and, on the third and fourth days 60 died. Many cattle were also killed.
An official investigating committee declared (42) that the symptoms were those of fluorine poisoning; but that only one plant was emitting fluorine and the amounts produced could not have caused the trouble. They said it must have been the sulphur dioxide and sulphuric acid.
However, van Leeuwen (43), Fenner (44), Flury (45), Teleky (46), and Schwartz (47) all disagreed. For one thing, windows and light bulbs showed etching by fluoride. Flury gave figures to show that toxic amounts of fluorine were present and Schwartz pointed out that soluble gases such as HF and SO, can become enriched in fog particles and produce acute poisoning even if the initial concentration is very small.
The evidence was carefully investigated by Roholm who was, at the time, the world”s greatest authority on fluorine poisoning. He said (48) that he was convinced by the symptoms and the details of the disaster that the malady was acute fluorine intoxication. Of the 27 factories in the region, 15 either used raw products containing fluorine (superphosphate works, zinc works) or add fluorine compounds to the raw materials (steel works, iron works, glass works), involving the possibility of passing gaseous fluorine compounds to the materials (steel works, iron works, glass works), involving the possibility of passing gaseous fluorine compounds (SiF4,k HF) into the chimney smoke.
Moreover, 20 years later the vegetation in the region contained enough fluorine to indicate tht fluorine pollution was high, and had probably been high in 1930 (49).
The Death-fogs, Denora, 1948 The next dramatic fog disaster was in Pennsylvania in 1948. The towns of Denora and Webster lie in a deep, narrow valley of the Monongahela River, shaped like a reversed letter “C” and tightly encolsed on all sides by hills rising four to five hundred feet above the river.
Within these narrow confines were zinc works, a steel plant with blast and open hearth furnaces, a wire mill, and two nail galvanizing mills. For years the neighbours had complained, chiefly of the most obvious pollutant which was sulphur fumes from the zinc plant. There were several successful damage suits for damage to health and property.
From October 27-31, a temperature inversion confined the pollution in an estimated 500 million cubic metres of trapped atmosphere. 6,000 of the 13,000 residents became ill, and on the fourth day 17 died. No one knows what would have happened if the fog hadn”t cleared the next day. Two more died that day, and another 8 days later, making 20 in all.
Moreover, recent investigations show (50) that those made ill have since had poorer health and a higher death-rate than those who were unaffected.
The Investigations The Steelworkers of America promptly donated $10,000 for an investigation and it was suggested that Dr. Kehoe be employed. However, Kettering Laboratories had already been retained by U.S. Steel to investigate.
Phillip Stadtler then investigated and reported both direct and circumstantial evidence of acute fluorine poisoning to people already suffering from chronic fluorine poisoning. The symptoms were those of fluorine poisoning; and blood-fluorine conentrations were 12 to 25 times normal. (51)
His investigation was followed by two others; one by Kettering Laboratory; and one by the Public Health Service.
The obvious ways to determine fluorine emission would be (a) by analysing surrounding vegetation; and (b) to analyse all materials going into the processes — ores, coal, coke, gas, limestone, fluorspar, etc, and all the products and recovered wastes. From the analyses the amounts could be calculated, and simple subtraction should be a fair measure of total fluorine emission.
This was done by Kehoe and his team (52) but his findings have never been published; and cannot be without the consent of U.S. Steel. It is fair to assume, however, that they would have been if they exonerated fluorine, since the zinc plant was the chief source of the other likely causes. Moreover, Kehoe has testified (53) that the principal hazard from steel manufacture is fluorine.
The PHS Approach The PHS approach was entirely different, and seems purposely designed to discover as little as possible. And that was the result. A 173 page report tells us (54) that there had been no unusual kind or amount of pollution, and that no pollutant present could have caused the trouble.
How the report can be taken seriously is past belief; but it is, and is generally accepted as the final word on the subject. It is an elaborate piece of hocus-pocus, wholly incompetent, irrevelant, and immaterial to prove anything except how easily people — and I mean those who call themselves scientists — can be duped.
Sampling methods of doubtful reliability were applied at arbitrarily selected times and places, and the results “averaged” with no attempt at proper weighting. Calculations therefrom, replete with arithmetical errors and discrepancies, were combined with outright guesses to arrive at estimates of emission.
They guess that 210 tons of coal burned in homes emit 30 lb. of fluorine but that 213 tons burned in the blooming-mill boilers emit only 4 lb. No possible reason for the difference is offered.
On page 104, waste gas from the blast furnace contains 4.6 mg of fluorine per cubic meter. On page 108 it contains one-tenth as much.
Calculations for open-hearth emission show a discrepancy of several thousand-fold, with no way to know where the error lies.
The “biological studies” and general air-sampling are similarly inappropriate and meaningless. Air samples at 12 arbitrarily selected points between Feb. 16 and April 27, 1949, can tell us nothing about concentrations during the episode.
PHS tells us that (55):
“There were few animal deaths near the smelter because farmers learned years ago the impossibility of healthful survival among destructive fumes.”
He believes the deaths were from sulphuric acid and perhaps they were. The point here is that the sole purpose of the PHS “study” was to whitewash fluorine as the cause.
The Death-fogs; London, 1952 The next major fog episode was in England, 1952. From December 5 to 9, the Thames Valley, and particularly London, were blanketed with fog confined by a temperature inversion. During the five days there were 2,000 excess deaths in London, and some 10,000 more in the surrounding area. This rates as the greatest fog disaster of all time, although 2,000 deaths among 8 million people are not nearly as many, proportionally, as 20 among 13,000 at Donora.
Again, it has been assumed that sulphur compounds, easily smelled, and known to have been present, were responsible. And again, there is little supporting evidence, and fluorine as a cause, or contributing cause, must be considered.
In an area the size of Michigan, England burns half as much coal as the entire United States. Moreover, it is densely populated with industries that pour out fluorine.
Bellingham, Washington, noted like London for its fogs, with heavy sulphur pollution from the pulp mills but with almost no fluorine, has never experienced fog-induced illness that could be noted.
On the other hand, the 1952 episode in London does not stand alone. A fog in 1945 brought 600 excess deaths, in 1956 500, and in 1957 400. (56) Since then there were two in rapid succession in 1959 (57); although one of these was probably complicated by a “flu” epidemic.
Prior to 1948, there had been fog episodes with illness in England in 1873, 1880, 1882, 1891, and 1892. But six in less than 11 years since 1948 would indicate a much greater awareness or an actual increase in the number. We might well wonder whether fluorine in the new fluoride-catalyzed gasolines may not be a factor.
But I am not here to prove that any of the three major death-fogs were due to fluorine. My interest is in the efforts to prove that they were not.
The Air-Pollution Studies After the London fog of 1952, there was much concern over air pollution in general and everyone got into the act. The Public Health Service saw a new field to exploit and started clamouring for appropriations. It still is.
Moreover, a rash of air studies began by people who couldn”t be trusted — people like air pollution control boards. So PHS and the Kettering Laboratory put on a fuorine study to end all fluorine studies.
In 1954 and early 1955, in co-opeeration with local or state health departments, it did fluorine sampling in 27 major cities. They took 24 hour samples once a week at from one to five points in each city — two in New York. In Anchorage, Pasadena, Washington, and Cincinnati they ran about a year. Some places they ran only a week. The average was 23 weeks. (58, 59)
“There may be no connection but, when total N.I.H. research grants in 1961 were increased 38 per cent over 1960, Washinton State University received 13 per cent less, and the University of Washington 94 per cent more, than their proportionate shares of the increase. The latter runs an ”Environmental Research Laboratory” but has made no study of fluorine since 1951.”
The Conferences There has also been a rash of conferences, national, international, and what have you, on both air and water pollution. I shall concern myself with two: the one in 1950, and the one in 1958.
Just a year after the Donora episode, President Truman asked the Secretary of Interior to organise an Interdepartmental Committee, which would sponsor a United States Technical Conference on air pollution. The conference was held in January, with Louis C. McCable of the Bureau of Mines as Chairman, and its Proceedings have been published. (60) There were seven panels: on agriculture, analytical methods and properties, equipment, health, instrumentation, legislation, and meteorology. J.G. Townsend, of PHS, and Robert Kehoe were co-chairmen of the Health Panel.
William Ashe, from Kettering, talked on the Donora incident. He offered nothing concrete, concurred with the PHS Report that there had been nothing there to cause what happened, and he even questioned if anything had really happened. The rest of the papers were of similar calibre.
The agriculture panel, on the other hand, was much concerned about fluorine, and some very valuable information was presented.
No such mistake was to be made at the National Conference on Air Pollution, called by the Public Health Service in 1958. There Dr. McCabe said: “The agriculture panel in 1950 was top heavy with fluorine studies because fluorides were in the news.”
And when Charles Butler, of the American Farm Bureau Federation twice brought up the farmer”s interest in fluorine pollution, the subject was quickly changed. (61)
When the conference was reported in Public Health Reports, the only mention of fluorine was in connection with Polk County Florida. It tells briefly of damage suits against the fertilizer industry, and of millions spent to control pollution. (62) One man was then suing eight fertilizer and chemical companies for more than a million in damages. (63)
PHS Now Wants to Help Us The latest thing is that PHS wants to be still more helpful; and Seattle has been chosen as the testing ground. A team of three has been sent, by invitation from our local health department, of course, to help with health problems that arise from suburban expansion. We are told that environmental health problems now transcend existing political boundaries, and will soon be of interstate and even international scope.
We are told (64) that the present mission is in support of the community effort; but that the principles and criteria arrived at will be applicable elsewhere. We will be helped to solve our problems as to water supply (we have beyond doubt the purest and most plentiful in the country) sewerage, air pollution, radiological health, food sanitation, and solid refuse collection and disposal.
“We are looking for gaps and areas that need de-emphasis.” The latter applies to fluorine of course. And we are told (and I quote): “We will try out some of our thinking on you folks.”
One of the three is Herbert Dunsmore, air pollution engineer of Alleghany County, which includes Pittsburgh, and former environmental health director of that county.
I asked him why they had never analysed Pittsburgh air for fluorine. He said they have no fluorine problems in Pittsburgh.
I asked him how he knew if he never looked; and I showed him the PHS Registers of Air Pollution Analyses, which list no fluorine analyses ever done in Pittsburgh. (58,59)
And we know without any air analyses that Pittsburgh has a major fluorine problem. And the biggest problem is to keep people from knowing about it.
In 1948, Churchill (39) reported fluorine up to 269 ppm in vegetation grown within 10 to 30 miles of Pittsburgh. It could only have come from the air. Both he, and Largent of the Kettering Laboratory, (65) blamed it on coal smoke. They may be largely right; but why not also on the steel industry.
Conclusion These are the people that the PHS wants to have help us with our pollution problem. Perhaps, like the Trojans, we ought to “Beware of the Greeks bringing Gifts.” The Trojans failed to heed a warning; and the “gift” was the famous Trojan Horse.
Perhaps I can best conclude by quoting a statement by Dr. James P. Dixon, Health Commisioner of Philadelphia. At the 1958 Air Pollution Conference, he said (66): “If gas masks are not to become as common in a hundred years as shoes are today in the civilised world, we should do well to heed our somewhat submerged instincts of self presservation and remember that — whatever other uses man may devise for it — air is essentially for breathing.”
And I would add that water is essentially for drinking.
[Frederick B. Exner lived in Seattle when he wrote this.]
References
1. Exner, F.B., Fluoridation, Part III, Northwest Medicine 54:1255-1269, (Nov.) 1955, pp. 1255-1256. Also: Exner, Waldbott and Rorty, The American Fluoridation Experiment, Devin-Adair, N.Y. 1955 (Revised 1961) pp. 119-121.
2. Exner, F.B. Testimony on Fluoridation of Public Water Supplies Before the Councils of American Medical Association. Also: Supplemental Testimony and Commentary, Jr. of Applied Nutrition, 12: No. 1, 1959, pp. 25-46.
3. Ost, H., The Fight Against Injurious Industrial Gases. Ztschr.Angew. Chem. 20:1689-1693, 1907.
4. Our Children”s Teeth, Comm. to Protect our Children”s Teeth, New York 1957.
5. Public Health Service Grants and Awards by the National Institutes of Health, Fiscal Year 1960, Part 1 U.S. Dept. of H.E.W. Washington, D.C.
6. Transcript of Record in the cases of Reynolds Metals Co. vs. The Paul Martin Family Nos. 14990-14991-14992 in the U.S. Court of Appeals for the Ninth Dist. P. 960.
7. Ibid. pp. 965-966.
8. Ibid. pp. 966-967 & 1003.
9. Deposition of Dr. Francis Heyroth, in the case of Higgins et al. vs. City of Sante Fe, in the District Court of Santa Fe County, New Mexico, p. 31.
10. Reference 6, page 1015.
11. Ibid. p. 977, also: Largent, E.J. and Ferneau I.F., Exposure to Fluorides in Magnesium Founding, Jr. Ind. Hyg. & Tox. 26:113-116, (Apr.) 1944; Largent, E.J., The Urinary Fluoride Excretion among Men Employed in Alkylation Plants Using the Hydrogen Fluoride Process, Jr. Ind. Hyg. &: Tox. 29:53-55, (Jan) 1947; and Largent, E.J., Bovard, P.G., andHeyroth, F.F. Reontgenographic Changes and Urinary Fluoride Excretion among Workmen Engaged in the Manufacture of Inorganic ”Fluorides, Am. Jr. of Roent. & Rad. Ther. 65:42-48 (Jan.) 1951.
12. Ref. 9 (a) p. 45; (b) p.87; (c) p. 39; (d) p. 40; (e) p. 23.
13. Reference 2; and Exner, F.B., Affidavit for Injunction Suit at Terre Haute, Indiana, Privately Printed.
14. Anon. The So-called Copper Teeth of Cattle, Brit. Dent. Fr., 28:141-142, 1907.
15. Bartolucci, A. Interesting Casesof Osteomalacia in Cattle, Mod. Zool. 23: Pt. Sci. 194, 1912.
16. Roholm, Kaj, Fluorine Intoxication: A Clinical-Hygenic Study, Lewis, London, 1937.
17. DeEds, Floyd, Chronic Fluorine Intoxication, Medicine 12:1-60 (Feb) 1933.
18. Official Food and Drug Administration Tolerances for Residues of Pesticide Chemicals, Nat. Agric. Chemicals Assn. News and Pesticide Rev. 15:5-14 (May) 1957.
19. Sollman, T., Schetter, O.H., and Wetzel N.C. Studies of Chronic Intoxication in Albino Rats, Jr. Pharm. Exp. Ther. 17:197.
20. Feltman, R. and Kosel, G., Fluoride in Pharmaceutical Preparations, North-west Medicine, 55:663-664 (June) 1956.
21. Same as 16, above.
22. Moller, P.F. and Gudjonsson, S.V. Massive Fluorosis of Bones and Ligaments, Ugr. Laeger, 95:1-9, 1933.
23. Shortt, H.E., et al., Endemic Fluorosis in the Madras Presidency, Ind. Jr. Med. Res. 25:553-568 (Oct.) 1937.
24. Cox, G.J., New Knowledge of Fluorine in Relation to Dental Caries, J.A.W.W.A., 31:1926-1930. (Nov.) 1939.
25. Stokinger H.E. and Woodward, R.L., Toxicologic Methods for Establishing Drinking Water Standards, J.A.W.W.A., April, 1958, p. 518.
26. Dean, H.T. et al., Domestic Water and Dental Caries, 11 Pub. Health Rep. 56:756-792, (April 11) 1941, p. 762.
27. Tampa Tribune, Sept. 15, 1961.
28. Leone, N.C. in Reference 4, p. 34.
29. Call R.C. et al., The Effect of Atmospheric Fluorides on Man. Summary or Final Technical Report IV (mimeo) Also: Ditto, Progress Report, Sep. 1, 1957 to Aug. 31, 1958 (mimeo).
30. Butler, C., Discussion in: Proceedings: Nat”l. Conf. on Air Pollution, Nov. 18-20, 1958, p. 268.
31. New York Times, Now, 10, 1957.
32. Reference 6, p. 443.
33. The (Portland) Oregonian, Oct. 15, 1957.
34. Compton, O.C., Remmert, L.F. and Mellenthin, W.M., Comparison ofFluorine Levels in Crops Before and After Alumimium Factory Operations in Dalles Area, Misc. Paper 95, Aug. 1960, Ore. State Coll. Agric. Exper. Sta.
35. Personal communications from a Longview Dentist.
36. Callaham, J.R., Fluorine Industry Chem. and Met. Eng., 52:94-99 (Mar. 1945).
37. Neumark, H.R., Fluorine and Advanced Rocket Propulsion, Missiles and Space, July, 1961, pp. 18-21.
38. Christofano, E. and Harris, W.B., The Industrial Hygiene of Uranium Refining, Arch. Environ. Health, 1:438-460 (Oct. 1960).
39. Churchill, H.V., Rowley, R.J. and Martin, L.N., Fluorine Content of Certain Vegetation in a Western Pennsylvania Area, Anal. Chem. 20:69-71, 1948.
40. 1961 World Almanac.
41. Crossley, H.E., Fluorine in Coal, IV, The Industrial Significance of Fluorine in Coal, in Steam-Raising, Gas-Making and Brewing, Jr. Soc. Chem. Ind. (London) 63:342-347, 1944.
42. Firket, J. (Secretary), The Cause of the Symptoms Found in the Meuse Valley During the fogs of December 1930. Results of the Judicial Investigation Made by Messrs. Delahu, Schoofs, Mage, Batta, Bovy and Firket, Bull. Roy. Acad. Med. 11:682-741, 1931.
43. van Leeuwen, W.S., The Fog Disaster in the Industrial Region of South Luttich, Munch. Med. Wochschr. 78:1337-1347, 1936.
44. Fenner, G., Belgian Fog Disaster of December 3-4, 1930, Hospitalstid. 79:1337-1347, 1936.
45. Flury, F., The Cause of the Deaths during the Fog Catastrophe in the Meuse Valley, Gewerbehyg. 7:117-25, 1937.
46. Teleky, L., Fog and Catastrophes Caused by Fog, Arch. Gewerbepathol. Gebwerbehyg. 13:6-28, 1954.
47. Schwartz, F., Meuse Valley Fog Catastrophe and Air-Raid Precautions, Protar 4:53-55, 1938. (Chem. Abstr. 35:6013, 1941).
48. Roholm, K., The Fog Disaster in the Meuse valley 1930; A Fluorine Intoxication, Jr. Ind. Hyg. Tox. 19:126-137, 1937.
49. Went, F.W., Global Aspects of Air Polutions as Checked by Damage to Vegetation, Proc. 3rd, Nat”l Air Pollution Symposium, Pasadena, 1955: 8-11.
50. Burney, Leroy E., Surg. Gen. PHS, Status Report to the Nation, Proc. Nat”l Conf. on Air Pollution, Nov. 18-20, 1958: 1-6 (p. 3), PHS, Washington, D.C.
51. Fluorine Gases in Atmosphere as Industrial Wastes Blamed for Death and Chronic Poisoning of Donora and Webster, Pa. Inhabitants, Chem. Eng. News, 26:3692, 1948.
52. Ashe, W.F., Acute Effects of Air Pollution in Donora, Pennsylvania, Air Pollution: Proc. of the U.S. Tech. Conf. on Air Pollution, Sponsored by the Interdepartmental Comm. on Air Pollution, Louis McCabe, Chairman, McGraw-Hill, N.Y., 1952, pp. 455-461.
53. Reference 6, supra. p. 1006.
54. Schrenk, H.H., Heinmann, H., Clayton G.D., Gafafer, W.M. and Wexler, H., Air Poluution in Donor, Pa. Epidemiology of the Unusual Smog Episode of October 1948. Preliminary Report, Pub. Health Bull. No. 306, Washington, D.C., 1949.
55. Mills, C.A., Air Pollution and Community Health, The Christopher Publishing House, Boston, 1954.
56. Carey, G.C. Chronic Bronchitis in Relation to Air Pollution in Great Britain, Proc. Nat”l Air Pollution Conf., 1958, PHS, Washington, D.C. pp. 203-207, Air Pollution, Published on behalf of the World Health Organisation, Columbia University Press, N.Y., 1961, p. 175.
57. London, Associated Press, Jan. 30, Feb. 19, Feb. 21, 1959.
58. Register of Air Pollution Analyses, U.S. Dept. of H.E.W., P.H.S. (As of January 1, 1956, Washington, D.C., 1958).
59. Register of the Air Pollution Analyses, U.S. Dept. of H.E.W., P.H.S., Jan. 1, 1956 — June 30, 1959, Wash. D.C. 1961.
60. See Reference 52, supra.
61. Butler, C. Impact of Air Pollution on our Economics — Agricultural Considerations, Proc. Nat”l Air Pollution Conf. 1958, P.H.S. Washington, D.C. 1958: 251-272. see pp. 254-255 and 268.
62. Public Health Reports, 74:420 (May) 1959.
63. Lakeland (Florida) Ledger, May 26, 1958.
64. Meeting of Health and Welfare Comm. Municipal League of Seattle and King County, 1961.
65. Largent, E.J., The Effects of Air-borne Fluorides on Livestock, Air Pollution: Proc. of U.S. Tech. Conf. McGraw-Hill, N.Y. 1952: 64-72.
66. Dixon, J.P., What Does Air Pollution Do to Humans? Proc. Nat”l Conf. on Air Polution, P.H.S. 1958, Washington, D.C., pp. 39-41.